The problem of plant resistance against bacteriosis pathogens is one of the most important in modern horticulture. In this regard, compounds increasing the stability of plant varieties by mobilizing their natural defense mechanisms are increasingly used, in particular salicylic acid (SA).
SA is one of the key molecules involved in the formation of immune response and systemic induced plant resistance against bacterial diseases. It is accumulated in infected areas of plants, transported by phloem and concentrated in remote uninfected leaves, where, in turn, takes place the expression of protective genes responsible for the structural and functional plant protection from stress [1]. SA participation in the mechanisms of plant resistance against diseases is associated with the induction of the generation of reactive oxygen species (ROS) and stimulation of the synthesis of a number of components related to the pathogenesis of plant components [2]. Among SA-dependent signaling systems there are NADPH-oxidase – SA, acting as an inhibitor of catalase activity and hydrogen peroxide accumulation regulator. NO-synthase – SA promotes NO-induced salicylate-dependent formation of mRNA protein PR1, and MAP-kinase – SA associated with the induction of the kinase isoforms SIPK and adenyl cyclase system [2].
Plant response to stress is accompanied by increased formation of AOS and activation of antioxidant system components. It is assumed that the interaction of AOS and antioxidants (AO) is an important part of signaling, which regulates gene expression and provides adaptive flexibility of the body. According to catalytic activity the components of the antioxidant system are divided into enzymatic and non-enzymatic. Enzymatic AO is characterized by high specificity of action that is directed against certain form of active oxygen, cellular localization and the use of catalysts mostly metals with variable valence (Fe, Mn, Cu) [3]. The main antioxidant enzymes are superoxide dismutase (SOD), catalase, peroxidase, enzymes of ascorbate-glutathione cycle, some transferase [4].
The group of non-enzymatic AO include ascorbic acid, glutathione, carotenoids, tocopherols, phenols, proline and sugars, peculiarity of which is their chemical diversity, lack of strict specificity with respect to the particular AOS, the ability to neutralize active radicals HO•, RO• and ROO•. For their elimination there are no such specialized enzymes like SOD or catalase, involving in cellular signaling and gene expression regulation, including controlling the antioxidant system [5].
Among the AO there can be found simple phenols, quinones, phenolcarbonic acids and their derivatives, flavonoids, catechins, and leucoanthocyanins [6, 7]. During plant growth, they accumulate in the cell vacuoles or polymerize into lignin, strengthening the secondary cell wall [6]. Phenolic compounds perform physiological functions, are involved in regulation of cell growth, cell wall formation, photosynthesis and respiration processes of plants [6]. Antioxidant properties of flavonoids are explained by their ability to serve as a “trap” for free radicals, and to chelate metal ions in radical processes [7].
SA induces resistance against diseases, causes increase in plant productivity, and its compositions are widely used in horticulture. With this in mind, the aim of this work was to study the influence of SA on the components of the antioxidant system in plants-regenerants of tomato varieties in the conditions of bacterial stress caused by bacterial spotting P. syringae pv. tomato.
Research methodology. The objects of research were in vitro cultivated plants-regenerants of tomato varieties Chaika and Malynovyi dzvin with different resistance to bacteriosis pathogens [8]. Plants-regenerants of tomatoes were cultivated in modified Murashige and Skoog growing medium supplemented by 0,4 mg/l of 6-benzylaminopurine, with the addition of salicylic acid in concentrations of 0,05; 0,1; 0,25; 0,5 and 1 mg/l (Fig. 1).
In experiments it was used the isolated in Dnipropetrovsk region strain P. syringae pv. tomato IS-28. In the experiments simulating the impact of stress factor to the basic growing medium it was added 4,0% inactivated cells of P. syringae pv. tomato IS-28 (titer of 20∙109 cells/ml) (IC) warmed up at 100° C during 2,5 hours.
Peroxidase enzyme activity in leaves of tomato plants was measured using spectrophotometric method according to the optical density of the reaction products formed by oxidation of benzidine per second for 120 s at wavelength
590 nm. Tissue sample weight of 200-300 mg was ground in cold porcelain mortar with cold pestle in 2 ml of acetate buffer (pH 5,0). The resulting homogenates was centrifuged for 5 minutes at 12 000 g. To store the samples were placed in a refrigerator at 4° C. The reaction mixture contained 150 mcl of 0,2 M Na-acetate buffer (pH 5,0), 150 mcl of 0,01% solution of muriatic benzidine of 50 mcl extract, 200 mcl of 0,3% hydrogen peroxide, and 200 mcl of distilled water. The control cuvette contained 150 mcl of 0,2 M Na-acetate buffer (pH 5,0), 200 mcl of 0,01% solution of muriatic benzidine, 50 mcl of extract, and 400 mcl of distilled
water [9].
Calculation of peroxidase enzyme activity was provided by the formula [9]:
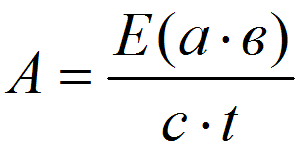
where E is an extinction = 0,125; a – the ratio of liquid taken for extract cooking, cm3/mg; B – the degree of constant dilution of extract in the reaction mixture; c – layer thickness, cm; t – time, s.
Soluble polyphenols were determined by Folin Ciocoalteu method in Singleton Rossi modification [10], based on the reaction of phenol with Folin-Chokalteu reagent. There is a mixture of phosphorus-tungsten and phosphorus-molybdenum acids recovering by oxidation of phenols to a mixture of oxides. Under these conditions it is produced a blue color, which is proportional to the amount of phenolic compounds, total contents of which was evaluated on a spectrophotometer Optizen POP (Korea). Plant material was extracted with 100% MEON at a ratio of 1:10. The amount of phenol was calculated by the formula:
F = (С×V extraction)/(m×1000),
where F – is the total content of intracellular phenolic compounds, mg∙g-1;C – is the concentration of phenolic compounds, l; V – extraction is the total volume of extraction, ml; m – is the mass of a sample, g.
The determination of flavonoid sum was performed with the help of spectrophotometric method with simultaneous analysis of a standard curve for quercetin [9]. The measurements were carried out in the presence of aluminum chloride and sodium acetate, forming stable complexes with flavonoids. Catechins were measured by spectrophotometric using 9 N H2SO4 and 1% vanillin with formation of stable complexes.
Antioxidant activity of phenols was established with the help of the modified method by Blois and Brand-Williams according to the assessment of antioxidant compounds and extracts. Spectrophotometric method based on the use of stable free radical 2,2-diphenyl-1-picrylhydrazyl, the essence of which is to reduce the optical density of the DFPH solution in the presence of antioxidants. Antioxidant activity was calculated by the formula: % inhibiting DFPH = 100•(Dк – Dо)/Dк,
where Dk is an optical density without antioxidants (control), Do is an optical density in the presence of antioxidants (for calibration curve – Trolox in certain concentrations) [9].
Research methodology. In terms of adding into the growing medium of SA in low concentrations, we found its growth stimulating effect on plants-regenerants of tomato varieties Chaika and Malynovyi dzvin under bacterial stress. Under these conditions, there was a significant increase in weight and length of internodes, shoots and roots (Table 1). The height of the plants-regenerants tomatoes in the control without SA use was 63,6 ± 0,5 mm. Thus, adding into the medium of 0,5 – 10 mg/l of SA under bacterial stress caused growth increase by an average of 7,7 – 20,0 % in a root and by 1,4 – 13,1 % in a stem. The most intense effect on plant height (Chaika variety – 73,2 ± 1,2 mm, Malynovyi dzvin variety – 68,4 ± 0,8 mm) was found in terms of SA use at a concentration of 1 mg/l. According to the literature [11] growth stimulating SA effect is caused by its impact on indoleacetic acid, which leads to increased mitotic activity, cell size and metabolic processes that underlie the processes of plant growth.
Increase in morphometric parameters of plants with increasing concentration of SA in the growing medium was not directly proportional. Thus, the effect of exogenous SA on tomato plants-regenerants depends on its concentration. In particular, high SA concentrations cause inhibition of mitotic activity, acidification of cytoplasm and changes in energy metabolism, which is one of the causes of DNA synthesis inhibition and cell proliferation [12].
The established stimulation of growth processes of plants was accompanied by quantitative changes in the components of the antioxidant system. Exogenous SA penetrating into cells activates a set of pro- and antioxidant systems, causing strengthening of adaptive responses to stressful environmental factors, meaning it can be used to develop environmentally friendly techniques to enhance the immune system of agricultural plants [13]. SA regulates cell protection system due to accumulation of phenolic compounds. It was shown that treatment plants with SA strengthened biosynthesis of phenolic compounds in the cells under the influence of phytotoxic compounds of tomato plant bacterial spotting pathogen. Under the action of 4,0 % of IC P. syringae pv. tomato IS-28 it increased the number of phenolic compounds in the leaves of tomato varieties from 29,5 to 32,7 %. Treatment of plants-regenerants with SA at concentrations of 0,5 – 5 mg/l, induced intensification of accumulation of soluble phenols, catechins and flavonoids under bacterial stress. In the leaves of plants-regenerants of tomato varieties Chaika and Malynovyi dzvin the maximum value of content of phenols was 15,11 – 17,00 mg/ml, of catechins – 26,17 – 28,29, and of flavonoids – 6,37 – 7,15 mg/ml in terms of adding 1 mg/l od SA. While acting of high concentrations of SA 5, 10 mg/l, the level of phenolic compounds was less when compared to the control, which, in our opinion, is due to the destruction of cell structures. Thus, the use of SA is one of the parts of a complex system, leading to increased resistance of plants against bacterial spotting of tomato, but the amount of SA cannot exceed a certain threshold concentration, which is required for activation of protection systems and optimal operation of the plant.
The influence of phytotoxic metabolites of bacteriosis pathogens on the structure of cell membranes is associated with the development of oxidative stress, caused by the increased formation of AOS, accompanied by damage to membrane lipids, nucleic acids and enzyme inactivation [13]. A key role in regulating the AOS amount in cells is played by an antioxidant protection system, the main function of which is to slow down and prevent intracellular oxidation of organic compounds, the implementation of protective actions to biological structures and detoxification of secondary metabolites [14]. It is shown that the antioxidant activity of phenols in the leaves of plants-regenerants of tomato varieties Chaika and Malynovyi dzvin in control was 5,54 and 4,72 mcIU-equiv in the medium with 4,0% IC P. syringae pv. tomato IS-28 – 10,44 and 9,56 mcIU-equiv. This is due to the level of AOS generation in these tomato varieties that have different levels of resistance against pathogenic bacteria. In the leaves of plants-regenerants in terms of joint activity of SA and phytotoxic metabolites of P. syringae pv. tomato IS-28, the antioxidant activity of phenols in Chaika variety increased by 4,94 – 7,04 mcIU-equiv when compared to the control, Malynovyi dzvin – 4,86 – 7,16 mcIU-equiv.
SA exhibits anti-stress effect and enhances the activity of the enzymes of superoxide dismutase peroxidase and catalase involved in AOS utilization [14]. In the study, the presence of phytotoxic metabolites caused a significant increase in peroxidase activity in the leaves of plants-regenerants in the variants in the MS medium with the addition of the SA into the medium (Fig. 3). Peroxidase activity in the control for Chaika variety was 27,4 ± 0,04 un.mh-1•s-1, for Malynovyi dzvin variety – 24,0 ± 0,04, in the medium with 4,0 % of IC P. syringae pv. tomato IS-28 36,2 ± 0,05 and 43,4 ± 0,03 un.mh-1•s-1, respectively. Under bacterial stress the maximum values of peroxidase activity in leaves of plants-regenerants was in a concentration of 1 mg/l of SA, which for Chaika variety was 94,5 ± 0,04 un.mh-1•s-1, and for Malynovyi dzvin variety – 81,16 ± 0,05 un.mh-1•s-1. The obtained data are consistent with studies conducted on potato plants infected with phytophthora. Using histochemical methods it was found a connection between the accumulation of phenolic compounds and peroxidase activity that correlated with increased resistance of plants [15]. The essential peroxidase protection property actually is an oxidation of phenolic compounds with hydrogen peroxide, accompanied with their polymerization into lignin and thereby increase in the strength of plant cell walls [16].
Conclusions. SA at a concentration of 1 mg/l identifies the most intense impact on growth and antioxidant system components of planst-regenerants of tomato varieties Chaika and Malynovyi dzvin. Treatment of plants-regenerants with SA caused accumulation of the number of soluble phenols, flavonoids and catechins in terms of bacterial stress. While the simultaneous action of SA and phytotoxic metabolites of P. syringae pv. tomato IS-28, the antioxidant activity of phenols in tomato variety Chaika increased by 4,94 – 7,04 mcIU-equiv, and of Malynovyi dzvin variety – by 4,86 – 7,16 mcIU-equiv. The increase of peroxidase antioxidant enzyme activity in leaves under SA confirms the effectiveness of its use for growth and activation of plant immunity for tomato under bacterial destruction.
- Linear characteristics of planst-regenerants of tomato varieties under effect of salicylic acid
| Variant | Length | Weight of plants, mg | |||
| IC P. syringae pv. tomato IS-28 |
Salicylic acid, mg/l | shoots, mm | roots, mm | internodes, mm | |
| Chaika | |||||
| – | – | 63,6±0,5 | 40,5±0,3 | 8,8±0,4 | 125±0,8 |
| 4,0 % | – | 49,9±0,6 | 37,8±0,3 | 8,2±0,2 | 112±0,7 |
| 4,0 % | 0,5 | 64,5±0,4 | 46,3±0,8 | 9,1±0,3 | 132±0,5 |
| 4,0 % | 1,0 | 73,2±1,2 | 50,6±1,0 | 10,3±0,3 | 150±1,1 |
| 4,0 % | 2,5 | 72,8±0,8 | 48,6±0,7 | 9,2±0,2 | 142±0,9 |
| 4,0 % | 5 | 70,1±0,3 | 46,7±0,5 | 9,0±0,2 | 138±1,0 |
| 4,0 % | 10 | 66,8±0,7 | 44,3±0,6 | 9,0±0,2 | 134±0,9 |
| Malynovyi dzvin | |||||
| – | – | 58,4±0,4 | 37,2±0,4 | 7,6±0,3 | 118±0,6 |
| 4,0 % | – | 44,2±0,4 | 36,4±0,4 | 7,2±0,5 | 103±0,6 |
| 4,0 % | 0,5 | 59,2±0,4 | 40,6±0,6 | 8,4±0,4 | 122±0,7 |
| 4,0 % | 1,0 | 65,4±0,8 | 45,8±0,5 | 9,2±0,4 | 138±0,6 |
| 4,0 % | 2,5 | 60,8±0,6 | 45,3±0,3 | 9,0±0,6 | 134±0,4 |
| 4,0 % | 5 | 59,2±0,4 | 40,9±0,4 | 8,0±0,3 | 128±0,3 |
| 4,0 % | 10 | 58,7±0,5 | 40,3±0,5 | 7,8±0,3 | 120±0,5 |
- The accumulation of phenolic compounds in the leaves of planst-regenerants of tomato varieties under effect of salicylic acid
| Variant | Phenols, mg/ml | Catechins, mg/ml | Flavonoids mg/ml | |
| IC P. syringae pv. tomato IS-28 |
Salicylic acid, mg/l | |||
| Chaika | ||||
| – | – | 6,72±0,07 | 4,61±0,03 | 4,56±0,03 |
| 4,0 % | – | 9,99±0,05 | 4,09±0,04 | 4,76±0,05 |
| 4,0 % | 0,5 | 10,16±0,06 | 10,72±0,05 | 4,25±0,07 |
| 4,0 % | 1,0 | 17,00±0,05 | 28,39±0,03 | 7,15±0,03 |
| 4,0 % | 2,5 | 12,86±0,04 | 15,43±0,08 | 5,62±0,05 |
| 4,0 % | 5 | 7,72±0,08 | 3,59±0,05 | 3,50±0,07 |
| 4,0 % | 10 | 6,71±0,04 | 3,38±0,03 | 1,79±0,07 |
| Malynovyi dzvin | ||||
| – | – | 5,23±0,07 | 3,77±0,06 | 3,86±0,05 |
| 4,0 % | – | 7,42±0,05 | 3,99±0,04 | 4,12±0,03 |
| 4,0 % | 0,5 | 8,36±0,05 | 9,63±0,04 | 4,37±0,05 |
| 4,0 % | 1,0 | 15,11±0,03 | 26,17±0,03 | 6,37±0,03 |
| 4,0 % | 2,5 | 10,82±0,07 | 13,88±0,06 | 4,83±0,04 |
| 4,0 % | 5 | 6,48±0,07 | 3,25±0,05 | 3,12±0,06 |
| 4,0 % | 10 | 5,22±0,02 | 3,12±0,03 | 2,88±0,03 |

Fig. 1.
The effectiveness of salicylic acid on growth of planst-regenerants of tomato varieties Chaika in terms of bacterial stress: А – 4,0 % of IC P. syringae pv. tomato IS-28, B – 1 mg/l SA + 4,0 % of IC P. syringae pv. tomato IS-28.
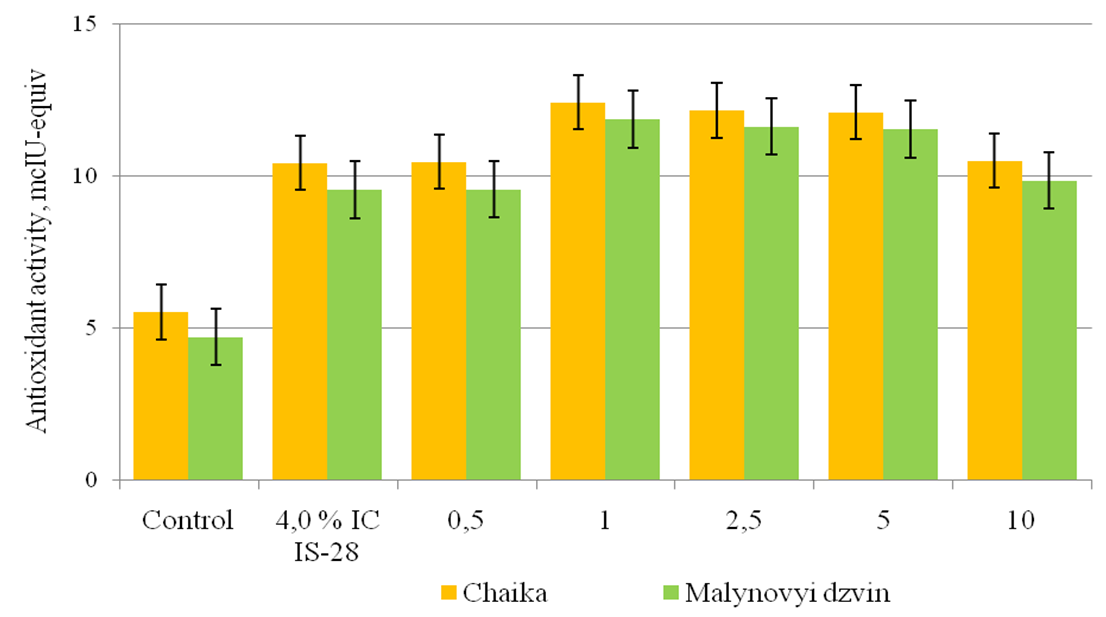
Fig. 2. The effectiveness of salicylic acid on antioxidant activity of phenols in the leaves of plants-regenerants of tomato varieties in terms of bacterial stress
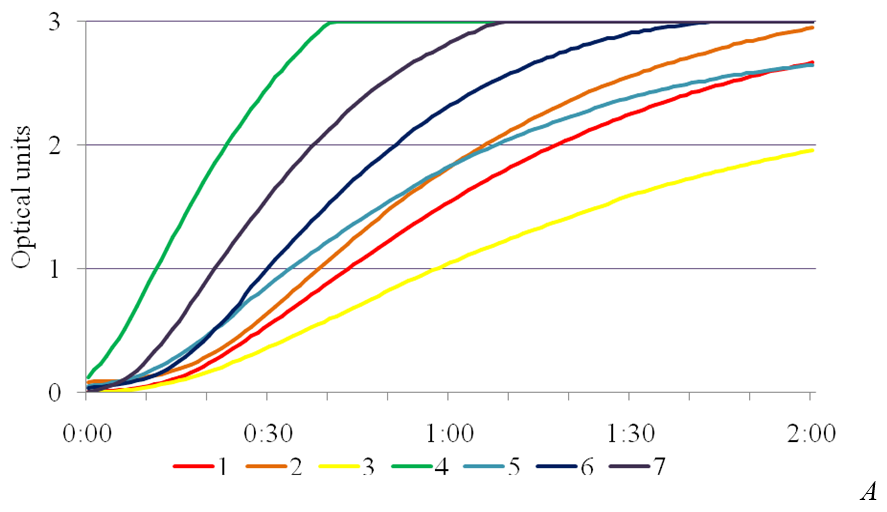
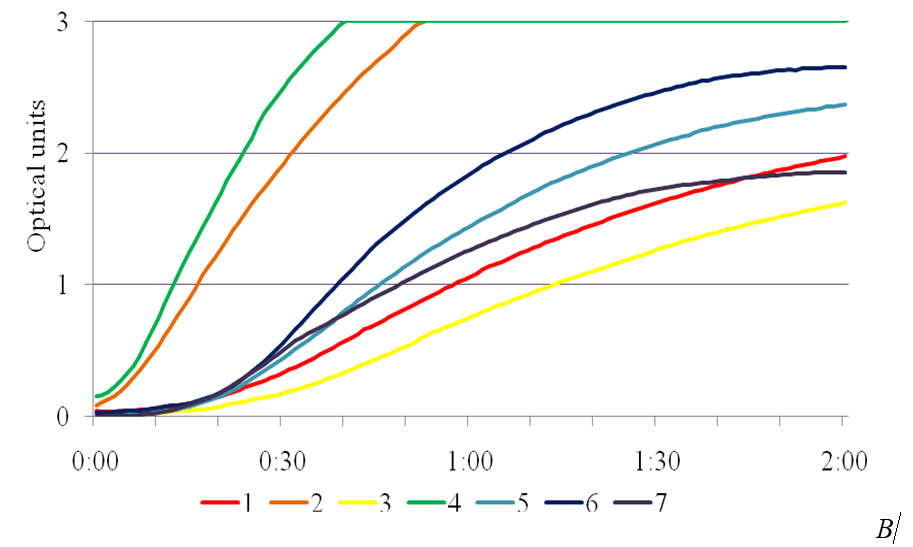
Fig. 3. The effectiveness of salicylic acid on activity of peroxidase in the leaves of plants-regenerants of tomato varieties Chaika (A) and Malynovyi dzvin (B) in terms of bacterial stress: 1 – 10 mg/l SA, 2 – 4,0 % IC IS-28, 3 – control,
4 – 1 mg/l SA, 5 – 5 mg/l SA, 6 – 0,5 mg/l SA, 7 – 2,5 mg/l SA.